Update as of May 12, 2023
Yesterday, the advisory committee voted to recommend the approval of neffy®, nasal epinephrine by ARS Pharmaceuticals, Inc., for both adults and children. The FDA is expected to make a decision on approval later this year. Watch our blog for more updates.
Asthma and Allergy Foundation of America (AAFA), testified before the Food and Drug Administration’s (FDA) Pulmonary-Allergy Drugs Advisory Committee (PADAC).
The PADAC is currently reviewing a new drug application submitted by ARS Pharmaceuticals Inc. for epinephrine nasal spray.
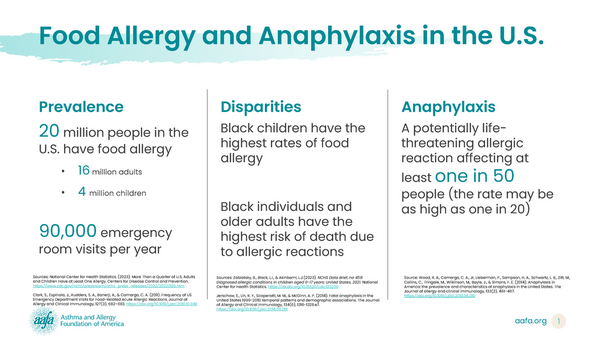
The medicine epinephrine is critical for the treatment of a severe allergic reaction called anaphylaxis. People with food allergies – as well as allergies to substances like drugs, insect bites and stings, and latex – must have epinephrine with them at all times. For decades, the only option for giving epinephrine has been with a shot. (Epinephrine can be injected with an auto-injector, a pre-filled syringe, or by using a needle to draw up the medicine from a vial.)
In her testimony, Carver shared data from AAFA’s My Life with Food Allergy Report to highlight why people with allergies need other epinephrine options, like a nasal spray.
In 2019, AAFA and its food allergy division, Kids with Food Allergies (KFA), conducted a three-part food allergy study titled “My Life With Food Allergy” to capture the patient and family experience with food allergies and anaphylaxis. We surveyed more than 2,200 people who either have food allergies, are parents of children with food allergies, or both.
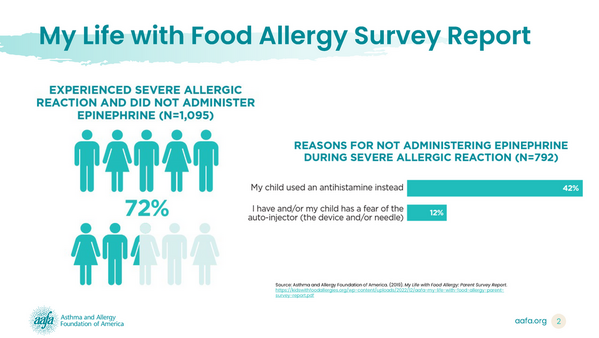
Around 90% of the parents surveyed in the My Life with Food Allergy report said their children had experienced at least one severe allergic reaction. Of these parents:
- Nearly three out of four said their child did not get epinephrine to treat the severe allergic reaction
- 42% opted to use an antihistamine instead (which is less likely to reverse the life-threatening symptoms of anaphylaxis)
- 12% stated that fear of the injection was a reason for not using epinephrine
As the FDA reviews the application, Carver encouraged the PADAC to consider that nasal epinephrine may make delivery easier, remove the fear of needle-based injections, and possibly increase adherence and confidence among people managing life-threatening allergies.
AAFA hopes that if the FDA finds this nasal epinephrine to be safe and effective, it will promote expanded access to all who need it.
We will keep this blog updated with the most recent information about the nasal epinephrine approval process. You can also read the full letter AAFA submitted to the PADAC.
Sign up to receive KFA’s Advocacy Action Alerts!
Would you like to help us advocate on future issues that affect people with food allergies? Join our online community. Not only will you receive Advocacy Action Alerts, but you’ll also be able to talk with other people managing food allergies on our support forums. You’ll also get alerts about other news about managing your conditions, research, and more.




Comments (0)