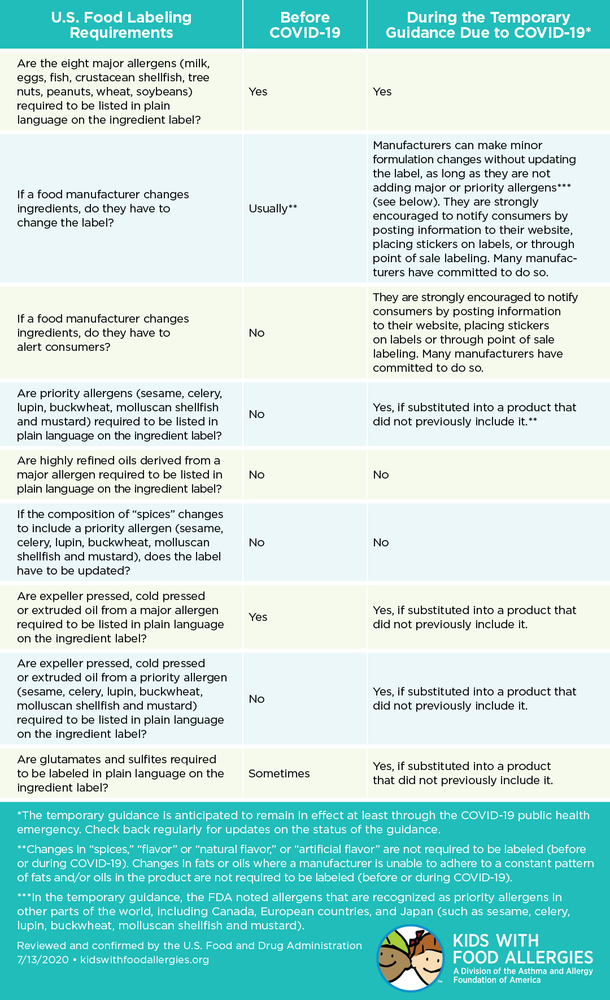
- Any substitution that involves a top 8 allergen recognized by law and those identified as “priority allergens” by other countries including sesame, celery, lupin, buckwheat, molluscan shellfish and mustard must require a label change by manufacturers and vendors
- The food industry has committed to limiting changes to rare situations and to communicating any substitutions made on a manufacturer’s website or at point-of-sale
- The guidance is set for the duration of the public health emergency but may need to be extended if food supply chains issues persist
This is a great food allergy advocacy win! Thank you to the FDA for listening to the needs of the food allergy community.
The new coronavirus pandemic (COVID-19) has impacted food supplies in many ways. As a result, the Food and Drug Administration (FDA) decided to temporarily change certain food labeling requirements to help food producers during this time, while addressing safety concerns. This change may affect some members of the food allergy community.
The Asthma and Allergy Foundation of America (AAFA) met with the FDA today to discuss the guidance the FDA gave to food manufacturers. We will continue to meet with the FDA and other advocacy organizations to address concerns. People with food allergies must be careful about avoiding foods they are allergic to in order to prevent reactions.
What Are the FDA’s Temporary Labeling Requirements?
Some ingredient supplies are impacted by the current pandemic. The costs of changing food labels can be in the millions and may be cost-prohibitive for food companies at this time. The FDA is giving food makers guidance on what types of ingredient substitutions are allowed or not allowed without updating the food label:
- Food producers may substitute certain minor ingredients without changing their ingredient labels.
- They are not allowed to substitute the top 8 food allergens (egg, milk, wheat, soy, peanut, tree nut, fish and crustacean shellfish) without updating the label or informing consumers.
- They are not allowed to substitute in other allergens (specifically sesame, celery, lupin, buckwheat, molluscan shellfish or mustard) without updating the label or informing consumers.
- They are not allowed to substitute other major ingredients that make up more than 2% of the food without updating the label.
- The FDA encourages food makers to alert consumers about changes in the ingredients in some way.
AAFA analyzed the FDA guidance with our legal and medical experts. (Read the full draft from the FDA .) We also created this chart with the help of the FDA to help you better understand the temporary labeling guidelines.
.) We also created this chart with the help of the FDA to help you better understand the temporary labeling guidelines.
U.S. Food Labeling Requirements
Before COVID-19
During the Temporary Guidance Due to COVID-19*
Are the eight major allergens (milk, eggs, fish, crustacean shellfish, tree nuts, peanuts, wheat, soybeans) required to be listed in plain language on the ingredient label?
Yes
Yes
If a food manufacturer changes ingredients, do they have to alert consumers?
No
They are strongly encouraged to notify consumers by posting information to their website, placing stickers on labels or through point of sale labeling. Many manufacturers have committed to do so.
Are highly refined oils derived from a major allergen required to be listed in plain language on the ingredient label?
No
No
Are expeller pressed, cold pressed or extruded oil from a priority allergen (sesame, celery, lupin, buckwheat, molluscan shellfish and mustard) required to be listed in plain language on the ingredient label?
No
Yes, if substituted into a product that did not previously include it.
Are glutamates and sulfites required to be labeled in plain language on the ingredient label?
Sometimes
Yes, if substituted into a product that did not previously include it.
*The temporary guidance is anticipated to remain in effect at least through the COVID-19 public health emergency. Check back regularly for updates on the status of the guidance.
**Changes in “spices,” “flavor” or “natural flavor,” or “artificial flavor” are not required to be labeled (before or during COVID-19). Changes in fats or oils where a manufacturer is unable to adhere to a constant pattern of fats and/or oils in the product are not required to be labeled (before or during COVID-19).
***In the temporary guidance, the FDA noted allergens that are recognized as priority allergens in other parts of the world, including Canada, European countries, and Japan (such as sesame, celery, lupin, buckwheat, molluscan shellfish and mustard).
Reviewed and confirmed by the U.S. Food and Drug Administration
Created 7/13/2020
Click to see larger image or share
To protect consumers with other less common food allergies, AAFA is asking the FDA to require food makers to make any substitutions public by sharing this information on the food company's website and social media.
"We recognize both the dire situation prompting this temporary guidance, as well as the FDA’s effort to include language specific to food allergy, including the top 8, as well as sesame and other increasingly prevalent allergies," notes Kenneth Mendez, CEO and president of AAFA."With a clear requirement that all substitutions be posted publicly, we feel confident that the risk to the consumer can be minimized to no more than baseline."
Before this temporary guidance was issued by the FDA, food labels were not required to disclose the addition of allergens like sesame and lupin. We have advocated for sesame to be formally included in the list of major allergens for several years.1,2
“Given the extraordinary circumstances we are in, I am pleased to see the FDA work to make food substitution safer,” says Dr. Mitchell Grayson, the chair of the Asthma and Allergy Foundation of America's (AAFA) Medical Scientific Council. “Food substitution likely has already occurred, so the overall risk to any patient is probably quite small. I’m heartened to see the FDA extending the list of allergens that cannot be substituted beyond the ‘big 8,’ which I believe shows the FDA’s concern for the food allergy community. AAFA has played an important role in working with the FDA to increase transparency on any food substitution. Overall, this is actually a win for the food allergy community, as the FDA guidance (with AAFA’s suggested additions) will allow for patients and families to determine those products that have had food substitutions and what substitutions occurred.”
What Do People With Food Allergies Need to Know About the FDA’s Announcement?
Key takeaways:
- The temporary food label guidelines will not allow foods to hide the top 8 most common food allergens.
- The guidelines also increase transparency on some other allergens (sesame, lupin, celery, buckwheat, molluscan shellfish and mustard).
- For other less common allergens, you may need to contact the food manufacturer to confirm ingredients — as you likely have been doing.
“The risk for a patient, as best as can be foreseen at present, is likely no more than at baseline,” says Dr. Matthew Greenhawt, member of AAFA’s Medical Scientific Council. “Concern for new exposures to allergens based on this new guidance statement is low. If manufacturers continue to follow FDA guidance, there should not be any anticipated issue regarding any substitutions, if ones are made. As always, patients should continue to follow avoidance strategies including label reading and discuss any products or concerns with their allergist.”
If you manage a food allergy that is not in the top 8 list, here are some steps you can take to help protect you or your child with food allergies:
- Talk to your allergist about this change and ask how they recommend you manage your food allergy during this time.
- Read every label every time. And if a food doesn’t have a label, don’t eat it or feed it to your child.
- Contact food companies to verify ingredients and possible substitutions.
- Watch the our blog and social media channels. We are asking the FDA to require food manufacturers to report any substitutions so people with food allergies have a way to find out that information. If the FDA agrees, we will announce it.
"It is important for every person with food allergy to understand exactly what this guidance means and if it pertains to their personal situation. While everyone relies upon clear and consistent messaging regarding ingredients, the types of substitutions outlined here will not increase risk for the vast majority of people with food allergies," explains Dr. David Stukus, member of AAFA's Medical Scientific Council. "For those who may be affected, it is especially important for them to discuss questions with their personal allergist to help understand their risk moving forward. Knowledge and understanding can increase confidence and decrease anxiety."
What Are the Next Steps?
AAFA has been advocating and will continue to advocate for food labeling laws that protect people with food allergies. We met with the FDA to ask them to require food companies to inform consumers about any substitutions by listing them on their websites and social channels. We have requested follow-up meetings with the FDA. We are in close communications with other food allergy advocacy organizations and are working together as a coalition to advocate for our community. We will keep you informed of any updates!
Posted on May 27, 2020. Updated June 19, 2020, and July 16, 2020
JOIN NOW
1. AAFA Sends Letter to FDA Requesting Sesame Labeling Discussion, December 18, 2019.
2. AAFA Signs Letter Supporting Regulations to Label for Sesame, December 21, 2018.

Comments (5)