On Sept. 13, 2019, the U.S. Food and Drug Administration’s (FDA) Allergenic Products Advisory Committee recommended the approval of Aimmune Therapeutics’ peanut treatment, Palforzia. If approved, Palforzia would be ground-breaking for the future of food allergy treatments.
What Is Palforzia?
Palforzia, also known as AR101, is a type of oral immunotherapy (OIT) for peanut allergy. If approved, it would be the first FDA-approved treatment for peanut allergy.
Currently, some allergists already offer OIT treatments. But there’s no set protocol or approved treatment.
Palforzia is a treatment used to reduce the incidence and severity of allergic reactions after accidental exposure to peanut in children ages 4 to 17 who have a confirmed diagnosis of peanut allergy. It is one of many treatments the FDA is exploring to fast track through the approval process to address an unmet need in the food allergy community.
How Does Palforzia Work?
Palforzia works by a process called “desensitization.” This involves very small amounts of peanut being given to the patient. Over time, the amounts are increased. Eventually, the patient will be able to tolerate larger amounts of peanut.
In the case of Palforzia, the patient may be able to tolerate up to two to three peanuts after several months of treatment. But for desensitization to work, the treatment must be continued indefinitely. Palforzia does not make peanut allergy go away. It only makes it less likely for accidental exposure to peanut to lead to a severe reaction.
Why Is Palforzia Important?
A food allergy can cause a severe allergic reaction called anaphylaxis. Around 2.5% of children have a peanut allergy. And between 2010 and 2017, the rate of peanut allergy in children grew 21%.1
There is currently no treatment for food allergies. Strict avoidance is the only way to avoid allergic reactions. And even with strict avoidance, accidental reactions are still too common.
A treatment like Palforzia could reduce the risk of a severe reaction due to accidental exposure. This could give many families more freedom and improve their quality of life.
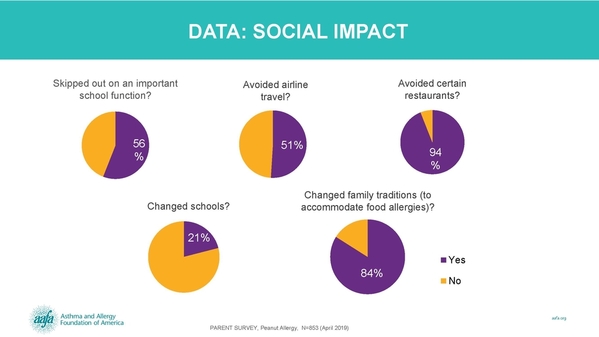
According to the Asthma and Allergy Foundation of America’s (AAFA) My Life With Food Allergy: Parent Survey Report, managing a child’s food allergy places a significant burden on the social, emotional and financial well-being of families. Many families limit eating out, travel, and going to school and social functions. As many as 84% have changed family traditions because of food allergies. After making a career change to care for their child with a food allergy, 81% saw a negative impact on their finances.
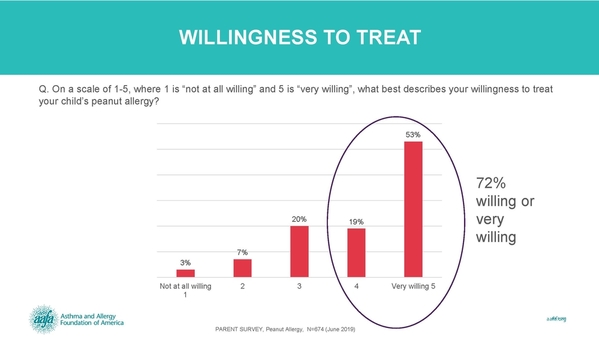
We also surveyed parents and caregivers of children with peanut allergy. We found that 72% of parents are willing to treat their child’s peanut allergy if a treatment is available.
Kenneth Mendez, AAFA’s CEO and president, shared these results with the FDA (download AAFA's presentation) on Sept. 13, and with the Institute for Clinical and Economic Review (ICER) in June, to make sure the patient voice was heard as they examine Palforzia and other peanut therapies.
What Was the Result of the FDA Committee’s Vote on Palforzia?
The FDA committee reviewed two questions on Sept. 13. On the question of, “Does Palforzia work?” the majority of the committee voted yes. On the question of, “Is it safe,” the majority also voted yes.
What Happens Next?
The FDA will continue their review of Palforzia and should have a decision within the next few months. Currently, we don’t know when this will be available or how it will impact anyone currently receiving OIT treatment for peanut allergy. But if Palforzia is approved, it could influence the future of other food allergy treatments.
What Is AAFA’s Position on Palforzia and OIT?
AAFA does not endorse treatments or medicines. Instead, our goal is to highlight the huge gap in the need for treatments for the food allergy community. Food allergies can place tremendous burden on families. Our community needs treatments that reduce the risk of allergic reactions and address quality of life. They need choices and access to treatments covered by insurance. Most of all, they need hope.
AAFA stood in for the millions of families managing food allergies at the FDA committee meeting. We presented our research, which painted the picture of what day-to-day life is like for families managing food allergies.
“We are deeply encouraged by the FDA’s decision to fast track new food allergy treatments as well as their effort to include patients and organizations in the drug approval process,” Mendez stated. “There has been a void of new breakthrough treatments in our community and having access to this therapy and similar therapies in the future will bring much needed peace of mind for families and patients living with food allergy.”
If Palforzia is approved, parents will need to work with their child’s doctors to decide if this is the right treatment for them. It does have side effects, including a risk of anaphylaxis.2 And until more studies are done, it is a lifelong treatment. Children who complete the treatment will likely have to stay on a maintenance dose indefinitely.3
What Are the Pros and Cons of Palforzia?
Palforzia has caused a lively debate among researchers and allergists for several reasons. The media has also been talking about it a lot during the past few days. Reuters, The Washington Post and The Atlantic have all published detailed articles on Palforzia and the FDA’s vote.
Since some allergists already offer non-FDA approved OIT, many feel it may increase the cost of OIT treatment. Some feel Palforzia cannot be tailored to individual patients and their experiences.
But approving Palfozia creates the chance for OIT to be covered under insurance. It would be more accessible for allergists. And it could make way for other treatments.
If approved, Palforzia may not be right for every child with a peanut allergy. But it could be a giant step toward a more hopeful and less stressful future all families managing food allergies.
Medical Review September 2019.
References
1. Gupta, R., Warren, C., Blumenstock, J., Kotowska, J., Mittal, K., & Smith, B. (2017). OR078 The prevalence of childhood food allergy in the United States: an update. Annals of Allergy, Asthma & Immunology, 119(5). doi: 10.1016/j.anai.2017.08.060
2. Chu, D. K., Wood, R. A., French, S., Fiocchi, A., Jordana, M., Waserman, S., … Schünemann, H. J. (2019). Oral immunotherapy for peanut allergy (PACE): a systematic review and meta-analysis of efficacy and safety. The Lancet, 393(10187), 2222–2232. doi: 10.1016/s0140-6736(19)30420-9
3. Few People with Peanut Allergy Tolerate Peanut after Stopping Oral Immunotherapy. (n.d.). Retrieved from https://www.niaid.nih.gov/news...g-oral-immunotherapy




Comments (0)